Concept Outline for a Pilot Observational Study of Deuterium‑Depleted Water in Pediatric Primary Immunodeficiency
Title: Deuterium-Depleted Water and B/T Cell Recovery in Pediatric Primary Immunodeficiency – Pilot Observational Study
Disclaimer and purpose of this document
This document has been prepared by JMD’s family as a non‑clinical concept proposal to support discussion with qualified clinician‑scientists at SickKids. It is not intended to function as a formal study protocol, nor to substitute for the expertise and judgment of medical professionals. Rather, it is offered as a suggested starting framework and summary of our thinking, with the understanding that any actual study design, methods, safety monitoring and analyses would be developed, modified or replaced entirely by the Principal Investigator and the SickKids research team in accordance with institutional standards and regulatory requirements.
1. Scientific rationale
Deuterium, a stable isotope of hydrogen, is hypothesized to influence cellular energy metabolism and immune function through effects on mitochondrial electron transport and reactive oxygen species (ROS) production. Deuterium-depleted water (DDW, <150 ppm deuterium vs. ~150 ppm in normal water) has been reported in some literature to support immune recovery and mitochondrial health, though clinical evidence in pediatric primary immunodeficiency (PID) is limited.
In children with FCHO1-related PID (IMD76) and other genetic defects of B or T cell development, recovery of lymphocyte numbers and function is critical. DDW, as a low-cost, seemingly low-risk nutritional intervention, may provide metabolic support for immune cell regeneration and activation and warrants formal pilot investigation.
2. Study objectives
Primary objectives
Assess feasibility and tolerability of DDW supplementation in children with PID over a 6–12 month follow-up period.
Characterize changes in absolute B‑cell and T‑cell counts (CD19+, CD4+, CD8+) and key functional subsets (naïve, memory, regulatory cells) by flow cytometry.
Secondary objectives
Assess changes in immunoglobulin levels (IgG, IgA, IgM, IgE).
Document infection frequency, antibiotic use, and clinical exacerbations.
Measure mitochondrial function markers and oxidative stress biomarkers (if available).
Assess quality of life and parent-reported outcomes.
3. Study design
Type: Prospective, single‑arm, observational pilot (N = 1–3 patients with confirmed PID).
Duration: 6–12 months of follow‑up per participant.
Setting: SickKids Immunology Clinic and SickKids Research Institute.
Intervention
Deuterium-depleted water at age‑appropriate volumes (~0.5–1 L/day, adjusted to body weight and tolerance).
Source and purity verified by supplier; deuterium content <150 ppm confirmed by mass spectrometry.
All participants remain on standard‑of‑care therapies (e.g., IVIG, prophylactic antibiotics).
4. Inclusion and exclusion criteria
Inclusion criteria
Confirmed primary immunodeficiency (genetic or phenotypic diagnosis).
Age 5–18 years.
Clinically stable on current therapies for ≥8 weeks prior to enrolment.
Parental/patient consent; age‑appropriate assent.
Exclusion criteria
Active infection or clinical deterioration within 4 weeks of enrolment.
Renal impairment (eGFR <60 mL/min/1.73 m²).
Pregnancy or breastfeeding (for adolescent females).
Inability to tolerate oral water supplementation.
Concurrent enrolment in other immunomodulatory interventional trials.
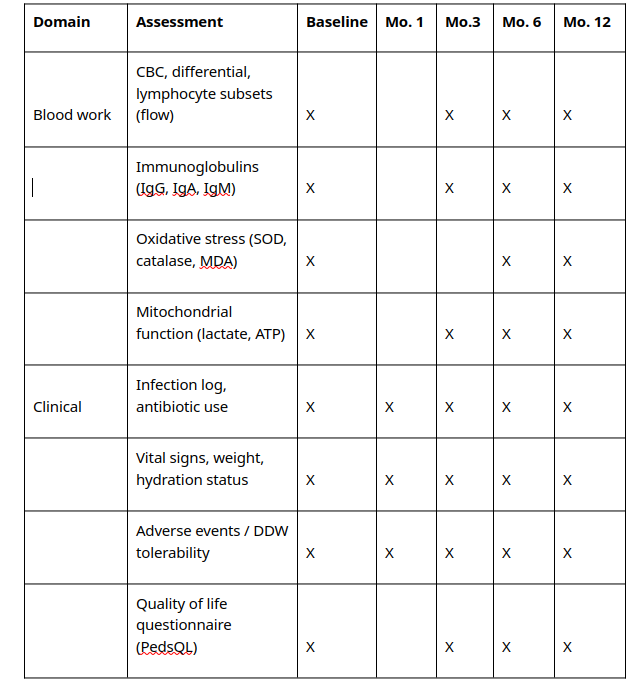
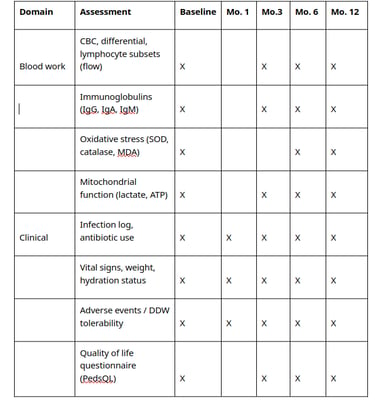
5. Study schedule and assessments
See below
6. Primary endpoints
Feasibility: ≥80% compliance with DDW intake over the study period; no serious adverse events attributable to DDW.
Immune recovery: Change in absolute B‑cell counts (CD19+) and absolute T‑cell counts (CD3+ CD4+, CD3+ CD8+), plus subset characterization.
7. Safety monitoring
Monthly phone contact for adverse‑event assessment.
Standard safety labs (electrolytes, renal function, liver function) at Months 3 and 6.
Ongoing review by the Principal Investigator and research team, with predefined escalation pathways for serious adverse events.
8. Data management and ethics
Data collected, managed, and stored in accordance with SickKids Research Ethics Board requirements and privacy regulations.
Participant identifiers stored separately from research data using coded IDs in secure, encrypted systems.
No identifiable information shared outside SickKids without explicit consent.
Results reported in aggregate; individual case descriptions only with specific consent.
Study to be registered on ClinicalTrials.gov prior to enrolment.
9. Statistical analysis
Descriptive statistics for baseline characteristics and longitudinal outcomes.
Graphical display of individual immune profiles (absolute counts, percentages, subset changes) over time.
Exploratory association between DDW adherence and immune changes if N ≥ 2.
Qualitative synthesis of safety and tolerability.
10. Publication and dissemination
Results to be submitted as a pilot or case series to a peer‑reviewed immunology or pediatric journal.
Annual lay summary to the funding partner through SickKids Foundation.
Institutional authorship model; funding partner acknowledged but without control over analysis or publication.
Any follow‑on, larger‑scale studies to follow the same ethics and transparency principles.
11. Budget estimate (12‑month pilot, single patient)
Item Estimated cost (CAD)
DDW supply (1 L/day, ~$5/L for 365 days) $1,825
Baseline & Month 12 flow cytometry (specialized immune subsets) $2,400Month 3 & 6 flow cytometry (standard subsets + selected assays) $2,000
Mitochondrial and oxidative stress assays $1,800
Immunoglobulin quantification $1,200
Research coordinator (0.25 FTE) $12,000
Data management, statistical analysis, quality assurance $2,500
Protocol development, REB submission, and documentation $2,000
Total (approximate) $25,725
Note: Costs will vary with actual enrolment, test menus, and institutional overhead. Figures here illustrate order of magnitude for a single‑patient 12‑month pilot; costs scale roughly linearly for 2–3 participants.
12. Contact
Prepared by: Frank Dionisi,
RAPID Foundation
Date: January 13, 2026
Version: 1.0 (January 2026)